Nacl Absorbance Spectrum
Next Previous Up Top 42 Identification without 3D Structure. NIST based solution at 2 mgml in 09 NaCl.

Sodium Chloride Nacl Optical Material
Osapublishing Org
1
There is very low ellipticity.
Nacl absorbance spectrum. Then the sample is fixed in the cell by skews and measured. 20 mgml in physiological water NaCl 09 Quality Control. 2 Check the spectrum of air to make sure the lamp is stable and the baseline is not drifting.
Using the same solution or a carefully diluted solution of it keeping track of the dilution you will need to determine the molar. The majority of the classical analytical methods rely on chemical reactions to perform an analysis. For volatile liquid sample.
There is a slight nonlinearity in the response pattern. 23209 or 23210 from. Lipoproteins and endotoxins has been confirmed using HEK-Blue TLR2 and HEKBlue TLR4 cells.
The absence of bacterial contamination eg. Infrared spectroscopy IR spectroscopy or vibrational spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption emission or reflectionIt is used to study and identify chemical substances or functional groups in solid liquid or gaseous forms. If it was determined by absorbance at 280 nm or a dye binding technique it could be too low.
It is used to detect different functional groups in PHB. FolinCiocalteau or Lowry method. And each protein has a distinct UV spectrum.
For example measuring the absorbance of a protein sample at 280 nm with a spectrophotometer is a rapid and straightforward method. The slope intercept and correlation coefficient R 2 of the absorbance versus the concentration was calculated by fitting the data to a linear equation using the least squares method. If there is a problem contact the manufacturer.
Whereas NaCl is transparent. The change in mass of potato disks incubated in water with that observed when the disks were incubated in saturated NaCl. The phenomenon of circular dichroism is very sensitive to the secondary structure of polypeptides and proteins Figure 21 and Figure 22 Circular dichroism CD spectroscopy is a form of light absorption spectroscopy that measures the difference in absorbance of right- and left.
A student measures the absorbance of a solution containing FeSCN2 ion using a spectrophotometer. The signal to noise is very low. When the Bradford reagent acidified Coomassie Brilliant Blue G-250 binds to proteins the dye undergoes a color change in the visible spectrum with the absorbance maximum moving from 470 to.
The desired result is to find out the absorbance of the dye and. Classical qualitative analysis is performed by adding one or a series of chemical reagents to the analyte. The assay with 25 ug sample giving an absorbance change of 0275 OD units.
Spectrum of a green solution it should show low absorbance of wavelengths in the green part of the spectrum but high absorption of light in the red and blue parts of the visible spectrum. Put a small amount 5-10 drops of the gold nanoparticle solution into 5 test tubes or microcentrifuge tubes. Live Chat Support.
Although your software will show higher absorbance values you should not trust to values higher than 2 absorbance units. It can be added in between two NaCl pellets. If necessary add additional water to the cuvette to get the absorbance on scale.
While the color of a substance depends on the person observing it absorption of specific wavelengths depends on the molecular structure of the substance. The main difference between the tests are the chemical groups which are responsible for the absorption or scattering of radiation eg peptide bonds aromatic side-groups basic groups and aggregated proteins. 421 Circular dichroism spectroscopy.
It was found that the absorbance of PDA-3coated CM was 94 and 88 in the UV 200 to 400 nm and visible 400 to 780 nm regions respectively. A home-built spectroelectrochemical setup was used to record steady-state absorbance spectra in the extended 5001400 nm range for voltages ranging from 01 to 06 V. Absorbance A is the logarithm to the base 10 of the reciprocal of the transmittance T.
The product BSA standard Nos. The ionic compounds NaCl and MgS are represented by the diagrams above. In contrast instrumental methods typically depend on the measurement of a physical property of the analyte.
The Molar Absorptivity Constant is specific for every single solution and at every wavelength. The absence of bacterial contamination eg. The Bradford protein assay is a time-tested colorimetric assay.
The absorbance values were corrected for the background absorbance of water or buffer and normalized to a 1 cm pathlength using the average pathlength of 100 μL of water 0568 0002 cm. Fourier transform infrared spectroscopy FTIR is a technique which is used to obtain infrared spectrum of absorption emission and photoconductivity of solid liquid and gas. All voltages were applied to the working electrode and are given versus Ag AgCl.
Spectrum is that of the flavoring agent vanillin shown below. We recommend to take in account absorbance values 0-1 or maximally 0-2. The source of the nonlinearity is in the reagent itself since there is an overlap in the spectrum.
You measured the absorbance of a dilute solution of indigotine at the same intervals across the spectrum and you blanked the spectrophotometer at. How the Bradford Protein Assay Works. Lipoproteins and endotoxins has been confirmed using HEK.
The peak at 17742 wavenumbers has the intesity of 0988 absorbance units. Run an absorption spectrum and record the absorbance at 280 nm. FTIR spectrum is recorded between 4000 and 400 cm 1For FTIR analysis the polymer was dissolved in chloroform and layered on a NaCl crystal and after.
Bovine serum albumin BSA. Use one tube as a color reference and add an equal volume of NaCl. If solvents are used to dissolve solids care must be taken to avoid obscuring important spectral regions by solvent absorption.
Check the concentration of the sample. The increase of absorbance at 595 nm is proportional to the amount of bound dye and thus to the amount concentration of protein present in the sample. The infrared spectrum above represents the absorption of certain wavelengths of radiation by molecules of CO2.
The background spectrum also takes into account several other factors related to the instrument. When you are taking an absorbance spectrum and measuring the absorbance at different wavelengths this is the only factor that is changing as the concentration of the solution remains the same and so does the pathlength. About the spectrum on picture 1 you can tell following.
This corresponds to 5 pg proteinml in the final assay volume. Alison Rodger Karen Sanders in Encyclopedia of Spectroscopy and Spectrometry Third Edition 2017. 5 mgml in physiological water NaCl 09 heated for 10 minutes at 65 - 70C Quality Control.
Citation needed Unlike other protein assays the Bradford protein assay is less susceptible to interference by various chemical compounds such as sodium potassium or even carbohydrates like sucrose that may be present in protein samples. Therefore the PDA-3based device could absorb majority of solar energy across the main spectrum. Absorbance spectrum can be produced.
Chemical analysis - chemical analysis - Classical methods. It can be used to characterize new materials or identify and verify known and unknown samples. Absorbance spectrum TLR3 activity has been verified using HEK-Blue TLR3 cells.
While the biuret method is sensitive in the range 05 to 25 mg protein per assay the Lowry method is 1 to 2 orders of magnitude more sensitive 5 to 150 μgThe main disadvantage of the Lowry method is the number of interfering substances. Absorbance spectrum TLR3 activity has been verified using HEK-Blue TLR3 cells. NaCl in deionized water as sketched in Figure1a.
The absorbance or turbidity of the solution being analyzed is then measured at the same wavelength and its protein concentration determined from the calibration curve.
Characteristic Spectra Of Energy Absorption For Dielectric Solids
Neb Com

Scielo Brasil Electrodeposition Of Gold Films From A Glycerol Solution On Carbon Paste Electrode And The Effect Of Chemical And Electrochemical Parameters Of Electrodeposition On The Electrode Performance In Potassium

Absorption Spectra Of 0 9 Nacl Solution And Pond Water In The Spectral Download Scientific Diagram
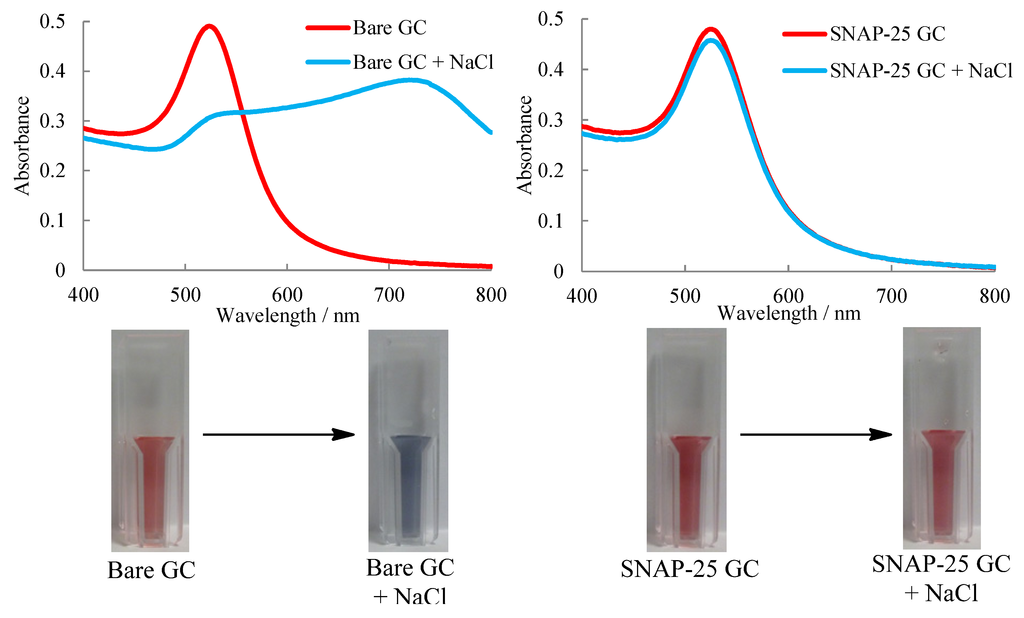
Toxins Free Full Text A Label Free Colorimetric Assay For The Detection Of Active Botulinum Neurotoxin Type A By Snap 25 Conjugated Colloidal Gold Html
Sodium Chloride
Journals Sagepub Com
Lucris Lub Lu Se
Comments
Post a Comment